Best Of The Best Info About How To Draw A Covalent Bond

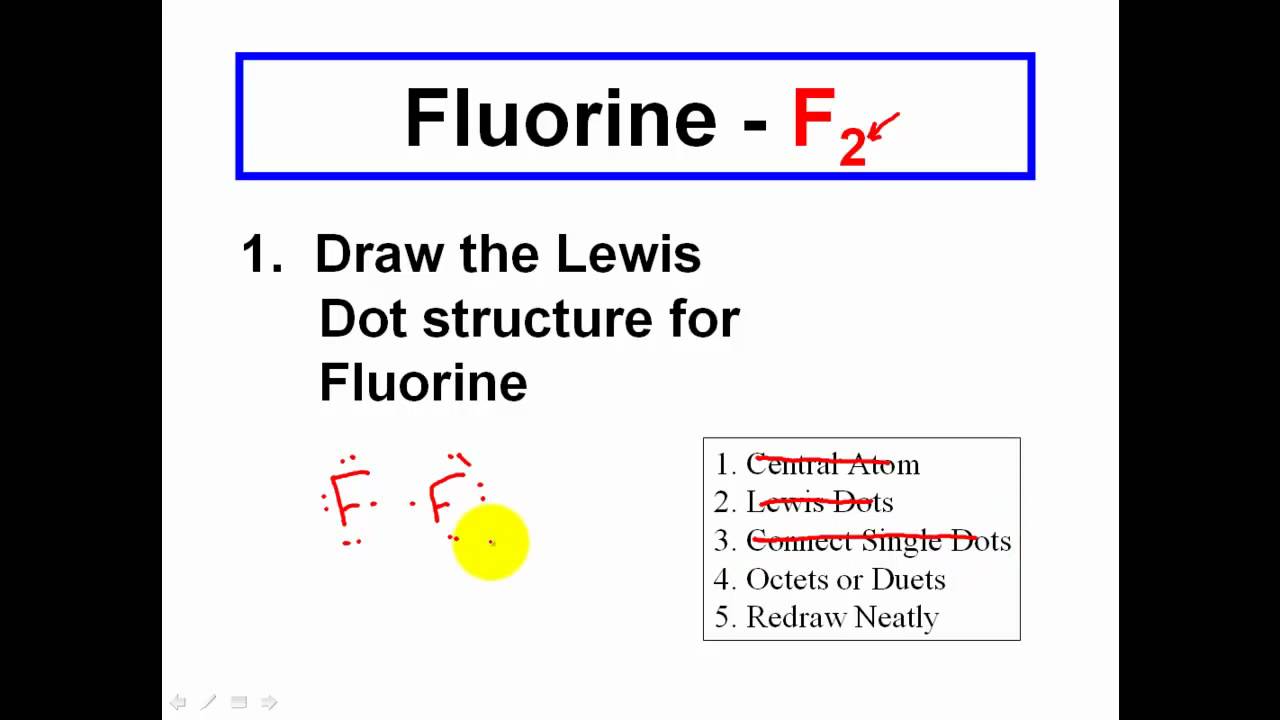
Draw a lewis dot structure for the valence shell of each element.
How to draw a covalent bond. Contents:0:08 introduction 0:39 h21:25 hcl2:23 cl2. You don't need to put a circle around the symbol for the nucleus. To determine how many leftovers.
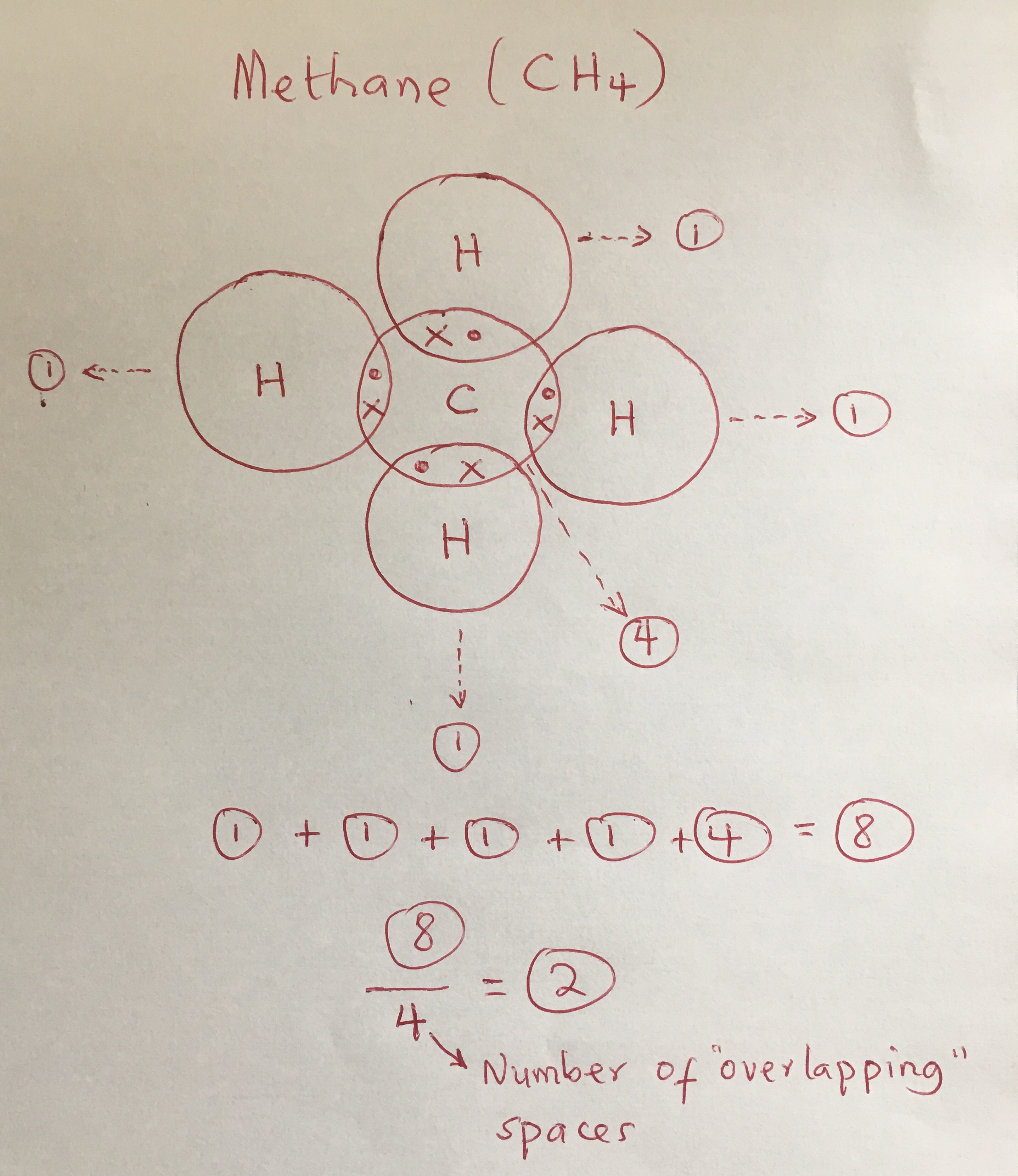
Given descriptions, diagrams, scenarios, or chemical symbols, students will model covalent bonds using electron dot formula (lewis structures). The sharing of electrons between atoms of different elements (for example h2o, ch4 and nh3 etc). Find the total number of valence electrons.
It is often easiest to draw circles at 90° or 180° to each other nitrogen is. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Determine the number of electrons necessary to satisfy the octet (or duet) rule with no electron sharing.
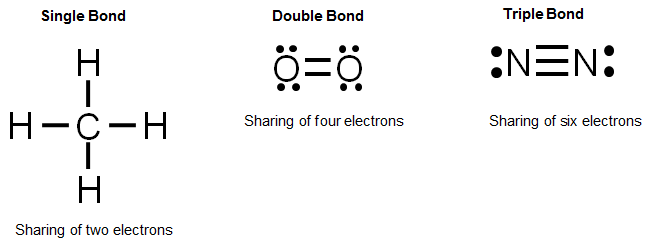
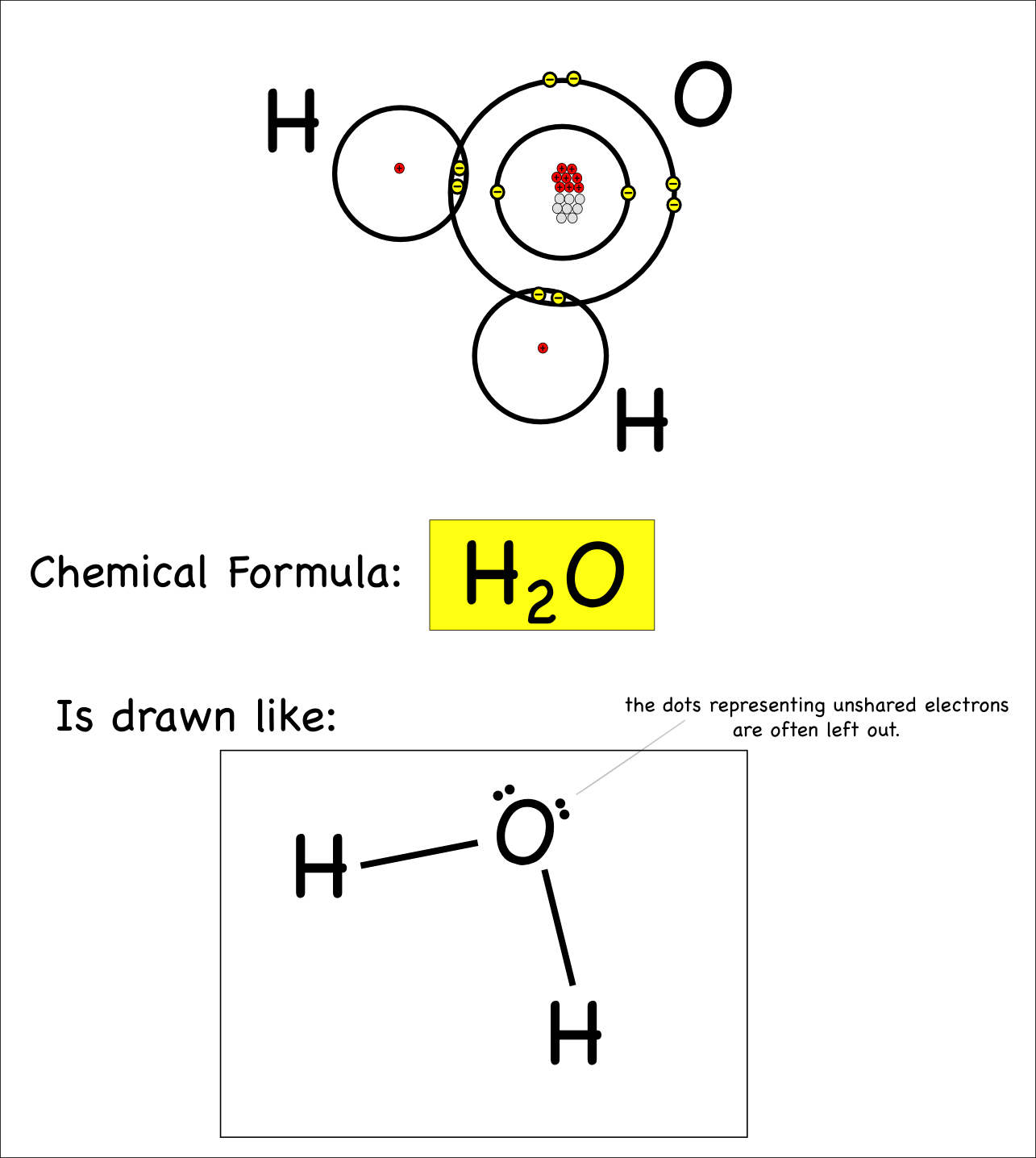
How do you draw a dot diagram for a covalent compound? Determine the number of valence electrons available. In this simpler type of diagram, the atoms are represented by writing their chemical symbols and a single covalent bond is represented by drawing a straight line between the two atoms.
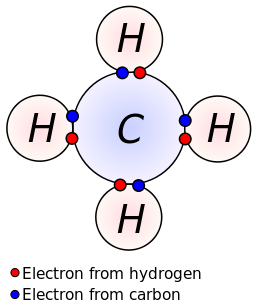
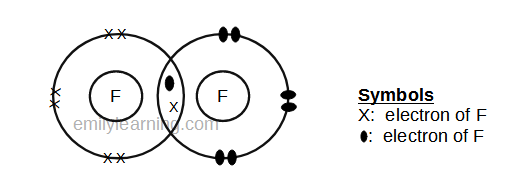
The two ways in which covalent bonding can be achieved: As covalent bonds are formed by sharing of electrons between atoms, we draw overlapping circles to show the overlapping of the electron shells, and draw in pairs of dots. Can the control room and power distribution room be built in the class a workshop?
In a lewis structure, atoms that are bonded covalently are. Each of the three h circles overlaps the n circle. What is a covalent bond in a lewis structure?